The minor isotope 6 Li is a potentially valuable nuclear source material for tritium production, an important component in hydrogen bombs, and a neutron absorber for the nuclear-fusion reaction. Lithium depleted in 6 Li may be distributed in commerce, with abundances of 6 Li as low as 2% and atomic weights in excess of 6.99. Its atomic number is 3. Its mass number is 6.94. It has two common isotopes, 6 Li and 7 Li. 7 Li is more common. 92.5% of lithium is 7 Li. Lithium is a soft silvery metal that is very reactive.
Knives Out is a great antidote to the Oscar contenders and family fare that dominate this time of year. It's a purely fun time at the movies thanks to its strong story and fantastic performances. Cast (in credits order) verified as complete. Ransom Drysdale. Cast of movie knives out 2020.

Lithium is a chemical element with symbol Li and atomic number 3. Classified as an alkali metal, Lithium is a solid at room temperature. Atomic Mass of Lithium Atomic mass of Lithium is 6.941 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). Atomic Weights and Isotopic Compositions for Lithium. Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 3: Li: 6: 6.015 122 8874(16) 0.0759(4) 6.
The major isotopes of lithium are (i) #'^7Li#, and (ii) #'^6Li#. The weighted average of these isotopes is #6.941##g*mol^-1#.
Explanation:
You will have to look up the isotopic distribution of lithium nuclei. ALL lithium nuclei have 3 positively charged nuclear particles, protons, i.e. #Z##=##3#. These 3 particles give rise to the element's identity. If you take the weighted average of ALL lithium nuclei, we get an average mass of #6.941##g*mol^-1#.
Related questions
Lithium, like the other alkali metals (Group I), is a sivery-white metal of very high chemical reactivity. It is less dense than water and will react actively with a water surface.
The minor isotope 6 Li is a potentially valuable nuclear source material for tritium production, an important component in hydrogen bombs, and a neutron absorber for the nuclear-fusion reaction. Lithium depleted in 6 Li may be distributed in commerce, with abundances of 6 Li as low as 2% and atomic weights in excess of 6.99. Its atomic number is 3. Its mass number is 6.94. It has two common isotopes, 6 Li and 7 Li. 7 Li is more common. 92.5% of lithium is 7 Li. Lithium is a soft silvery metal that is very reactive.
Knives Out is a great antidote to the Oscar contenders and family fare that dominate this time of year. It's a purely fun time at the movies thanks to its strong story and fantastic performances. Cast (in credits order) verified as complete. Ransom Drysdale. Cast of movie knives out 2020.
Lithium is a chemical element with symbol Li and atomic number 3. Classified as an alkali metal, Lithium is a solid at room temperature. Atomic Mass of Lithium Atomic mass of Lithium is 6.941 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). Atomic Weights and Isotopic Compositions for Lithium. Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 3: Li: 6: 6.015 122 8874(16) 0.0759(4) 6.
The major isotopes of lithium are (i) #'^7Li#, and (ii) #'^6Li#. The weighted average of these isotopes is #6.941##g*mol^-1#.
Explanation:
You will have to look up the isotopic distribution of lithium nuclei. ALL lithium nuclei have 3 positively charged nuclear particles, protons, i.e. #Z##=##3#. These 3 particles give rise to the element's identity. If you take the weighted average of ALL lithium nuclei, we get an average mass of #6.941##g*mol^-1#.
Related questions
Lithium, like the other alkali metals (Group I), is a sivery-white metal of very high chemical reactivity. It is less dense than water and will react actively with a water surface.
Compounds of lithium have been used in the manufacture of glass and of glazes for dishes and porcelain objects. It is used in lubricants, batteries and antidepressants.
Lithium finds use in nuclear breeder reactors as a coolant and a source for tritium. Tritium is formed by bombarding lithium with fast neutrons. If deuterium-tritium fusion becomes viable as an energy source, then the scarcity of lithium from which to breed tritium would become one of the limitations on the energy resource.
Lithium is contained in the silicate minerals elbaite, lepidolite, sugilite, neptunite and bikitaite. Other lithium aluminum silicates are spodumene, LiAlSi2O9 and petalite, LiAlSi4O10. Lithium and sodium are found with either zirconium, titanium or hafnium in the mineral zektzerite
Lithium is found in the phosphate minerals amblygonite, triphylite and montebrasite.
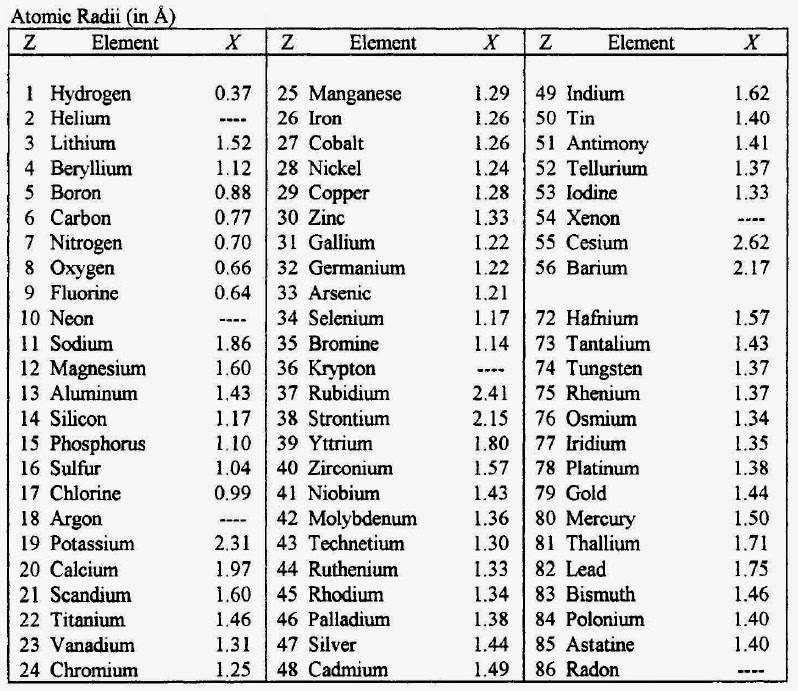
| Atomic data | Nuclear data |
Lithium Atomic Mass Ionic Bond
| Electron energy level diagram |

